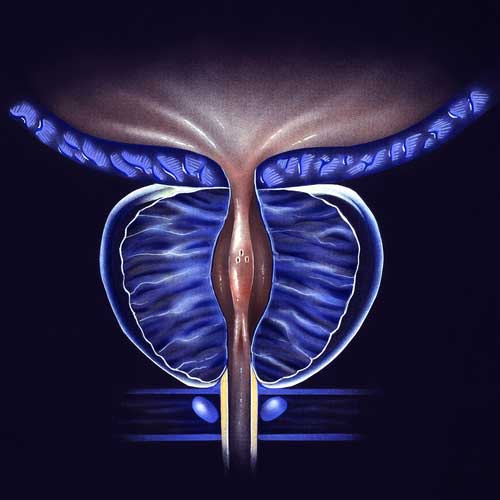
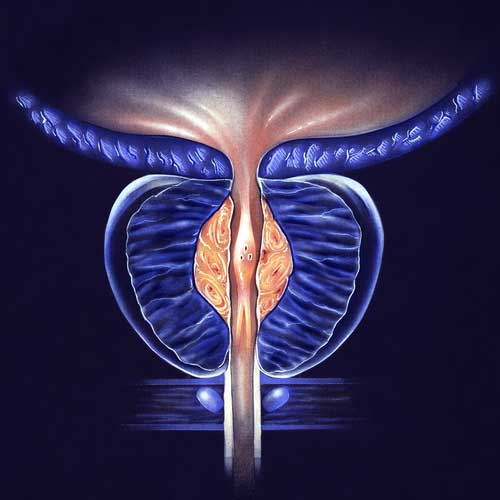
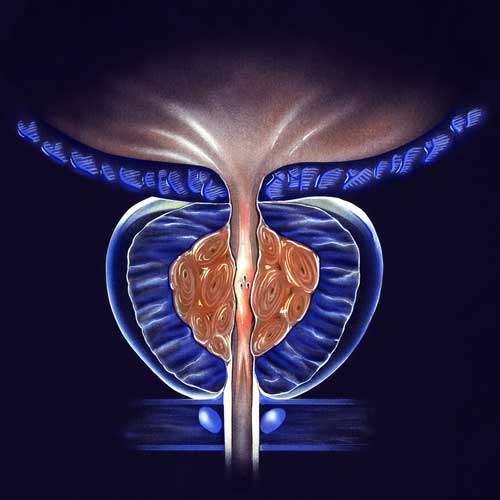
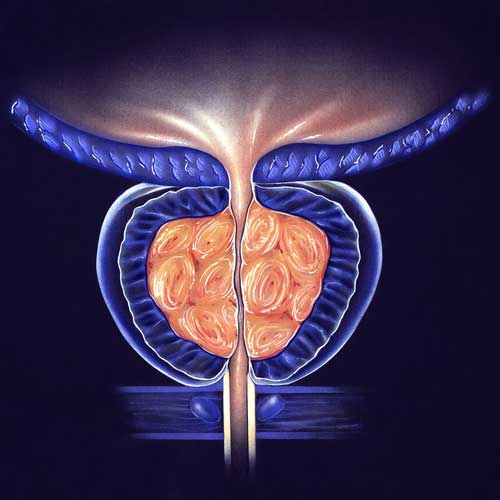
BPH/ENLARGED PROSTATE CLINICAL TRIALS
A Randomized, International Study to Assess the Safety of iTind Compared to UroLift (MT-08)
With the use of a minimally invasive surgery called UroLift, lower urinary tract symptoms (LUTS) brought on by benign prostatic hyperplasia (BPH) can be relieved. Regarding the improvement of objective and subjective measures, this treatment has demonstrated high efficacy. A temporary device known as the iTind uses minimally invasive surgery to treat LUTS that develops as a result of BPH. Comparing the minimally invasive iTind device to the UroLift device is the goal of this study.
General Requirements:
- Diagnosis of lower urinary tract symptoms presumed to be secondary to benign prostatic enlargement causing bladder outlet obstruction for which treatment is recommended
- Males ≥ 50 years of age or older
- Screening PSA < 4 mg/dl
- Prostate volume up to 75 cc documented by TRUS or MRI.
Customized TULSA-PRO Ablation Registry
Data from patients who have undergone or are currently undergoing the transurethral ultrasound ablation (TULSA) procedure as part of their regular clinical care will be entered into this patient registry. The registry will shed light on actual outcomes of procedure safety and effectiveness and help researchers determine how a patient's quality of life changes over the course of their lifespan and follow-up visits.
General Requirements:
- Male
- >18 years old
- Candidate for TULSA-PRO treatment
- willing and able to sign the Informed Consent form